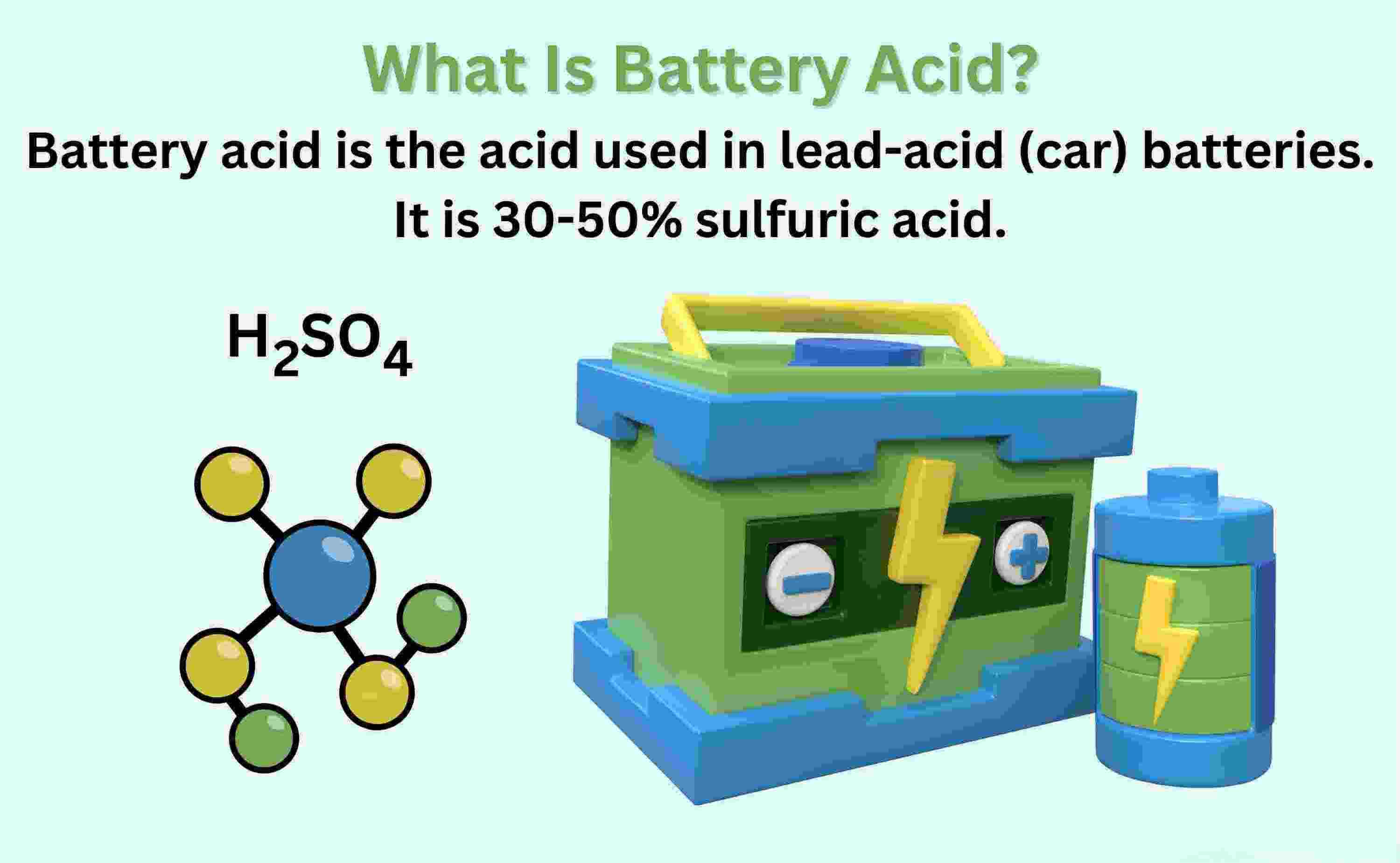
Battery acid, also known as electrolyte or sulfuric acid, is a highly corrosive and acidic substance found in lead-acid batteries. It plays a crucial role in the functioning of these batteries. The main component of battery acid is sulfuric acid (H2SO4). It is a strong acid that is highly reactive and capable of releasing hydrogen ions (H+) in solution. Battery acid is typically a solution of sulfuric acid diluted with water to achieve the desired concentration. The concentration of battery acid can vary depending on the type of battery and its intended use. In lead-acid batteries, the concentration of sulfuric acid is typically around 30% to 50% by weight. This concentration allows for efficient electrochemical reactions within the battery.

The pH of battery acid, which is primarily composed of sulfuric acid, typically ranges from around 0.8. This pH level indicates an extremely acidic nature. The pH scale is logarithmic, meaning that each unit represents a tenfold difference in acidity or alkalinity. Battery acid's low pH is a result of its high concentration of hydrogen ions (H+). Sulfuric acid is a strong acid that dissociates almost completely in water, releasing a large number of hydrogen ions. These hydrogen ions contribute to the acidity of the solution, lowering its pH value. The high acidity of battery acid serves important functions in lead-acid batteries. It helps facilitate the electrochemical reactions involved in the battery's charging and discharging processes. The acidic environment promotes the conversion of lead compounds on the electrodes, aiding in the storage and release of electrical energy.
In lead-acid batteries, the concentration of sulfuric acid in water typically varies from about 29% to 32% by weight. This translates to a molar concentration ranging from approximately 4.2 mol/L to 5.0 mol/L. The concentration of the sulfuric acid solution is often indicated by its specific gravity, with higher values signifying more concentrated solutions. For a fully charged lead-acid battery, the specific gravity is around 1.280, which corresponds to a sulfuric acid concentration of approximately 5.0 mol/L.
-Electrolyte: Battery acid acts as an electrolyte, providing the medium for the flow of ions between the battery's electrodes. It facilitates the chemical reactions that occur during both charging and discharging processes.
-Ion carrier: Battery acid carries charged ions between the positive and negative electrodes, allowing for the movement of electrons and the flow of electric current.
-Acidic environment: The acidity of battery acid helps in the formation and maintenance of the electrochemical reactions within the battery. It promotes the conversion of lead compounds into lead dioxide on the positive electrode and lead sulfate on the negative electrode.
-Density and specific gravity: The concentration of battery acid affects its density and specific gravity. These properties are important for measuring the state of charge and determining the battery's overall health.

During the charge and discharge process of a lead-acid battery, chemical reactions take place to store and release electrical energy. In a charged state, the cathode consists of lead dioxide (PbO2), while the anode is made of pure lead (Pb).
During charging, the lead dioxide undergoes an oxidation reaction, where it combines with the sulfuric acid electrolyte to form lead sulfate (PbSO4) and water (H2O). Simultaneously, the pure lead anode reacts with sulfate ions to also produce lead sulfate. This charging process converts the chemical energy into electrical energy, charging the battery.
When the battery is discharged, the reverse reactions occur. The lead sulfate on both the cathode and anode reacts with the sulfuric acid electrolyte to reform lead dioxide on the cathode and pure lead on the anode. This conversion of chemical energy back into electrical energy powers the device using the battery.
These reversible chemical reactions occur between the lead, lead dioxide, and sulfate ions in the presence of sulfuric acid electrolyte. By repeating the charge and discharge cycles, a lead-acid battery can efficiently store and deliver electrical energy for various applications.
Baking soda, also known as sodium bicarbonate (NaHCO3), is commonly used to neutralize battery acid. When baking soda reacts with battery acid (sulfuric acid), it undergoes a chemical reaction that helps neutralize the acid. The reaction produces carbon dioxide gas (CO2) and water (H2O), resulting in a less acidic or neutralized solution. It's important to note that when neutralizing battery acid, an appropriate amount of baking soda should be used to effectively neutralize the acid. The exact quantity may vary depending on the amount and concentration of the acid being neutralized. Other alkaline substances, such as lime (calcium hydroxide) or ammonia solution, can also be used to neutralize battery acid. However, baking soda is generally more readily available and commonly used for this purpose due to its effectiveness and ease of use.
Battery acid, which is typically a mixture of sulfuric acid (H2SO4) and water, is a highly corrosive substance commonly found in lead-acid batteries used in vehicles and various industrial applications. Here are some detailed hazards associated with battery acid.

To human health
-Chemical burns: Direct contact with battery acid can cause severe chemical burns on the skin, which can be painful and may lead to permanent damage if not treated promptly.
-Eye damage: If battery acid gets into the eyes, it can cause serious injury, including blindness, due to the corrosive nature of the acid.
-Respiratory issues: Inhaling fumes from battery acid can lead to respiratory problems, including coughing, wheezing, and difficulty breathing. Prolonged exposure may lead to more severe respiratory conditions.
-Ingestion: Ingesting battery acid is extremely dangerous and can cause severe internal burns, organ damage, and even death.
-Skin absorption: Prolonged exposure to battery acid, even without direct contact, can lead to skin absorption, which may cause systemic toxicity.
To the environment
-Water contamination: If battery acid is improperly disposed of and enters water systems, it can contaminate drinking water and harm aquatic life. The acid can disrupt the pH balance of water bodies, affecting the ecosystem.
-Soil acidification: Spills or leaks of battery acid can lead to soil acidification, which can harm plant life and reduce soil fertility.
-Air pollution: The release of sulfuric acid into the atmosphere can contribute to air pollution and acid rain, which has detrimental effects on forests, lakes, and buildings.
-Habitat destruction: The corrosive nature of battery acid can destroy habitats and ecosystems, particularly if it contaminates water sources.
If a battery has leaked acid, it's essential to clean it up carefully and safely to prevent harm to people and the environment. Here's a detailed step-by-step guide on how to clean up a battery acid spill.
-Ensure safety: Before starting the cleanup, ensure that the area is safe. Wear appropriate personal protective equipment (PPE), including rubber gloves, safety goggles, and a face shield or mask to prevent contact with the acid.
-Ventilate the area: Open windows and doors to ventilate the area and reduce the concentration of any harmful fumes.
-Neutralize the acid: Use a neutralizing agent to neutralize the battery acid. Baking soda (sodium bicarbonate) is a common household item that can be used for this purpose. Sprinkle a generous amount of baking soda over the spill. The reaction between the baking soda and the acid will produce a foamy substance, which is a sign that the neutralization is taking place.
-Absorb the neutralized material: Once the acid has been neutralized, use a non-absorbent material like kitty litter, sand, or a commercial absorbent product to soak up the neutralized material. Avoid using paper towels or cloth as they can become saturated and may not effectively absorb the material.
-Collect the absorbent material: Carefully collect the absorbent material that has soaked up the neutralized acid. Place it into a plastic bag or a designated container for hazardous waste.
-Clean the area: After the initial absorbent material has been removed, clean the area with a solution of water and mild detergent. Rinse thoroughly with water to ensure that all traces of the acid have been removed.
-Dispose of materials properly: Dispose of the used PPE, absorbent material, and any other contaminated items according to local regulations for hazardous waste disposal.
-Monitor for residual effects: Keep an eye on the area for any signs of residual effects, such as continued fumes or discoloration. If any issues persist, it may be necessary to contact a professional cleaning service or environmental health and safety officials.
-Wash hands and equipment: After the cleanup is complete, thoroughly wash your hands with soap and water. Clean any equipment or tools used during the cleanup process.
-Inform relevant parties: If the spill occurred in a workplace or public area, inform the relevant parties or management about the incident and the steps taken to address it.
It's important to remember that battery acid is a hazardous substance, and improper handling can lead to serious health risks and environmental damage. Always follow the manufacturer's guidelines and local regulations for handling and disposing of battery acid and related materials.
NEWARE TECHNOLOGY LLC
755 Ames Avenue, Milpitas, CA, USA, 95035










