Accumulator is also called rechargeable battery, is a type of battery that can be recharged, discharged, and reused multiple times. Unlike disposable batteries, which are intended for single use and cannot be recharged, accumulators offer the advantage of being able to undergo a charging process that restores their energy storage capacity.
Accumulators are designed with reversible chemical reactions that occur during charge and discharge cycles. When an accumulator is charged, electrical energy is converted into chemical energy, which is stored within the battery. This chemical energy can be released as electrical energy when the accumulator discharges. The ability to be recharged and discharged multiple times makes accumulators a more cost-effective and environmentally friendly option compared to disposable batteries.
One example of an accumulator is the lithium-ion battery, widely used in various applications such as portable electronic devices (e.g., smartphones, laptops), electric vehicles, and renewable energy systems. Lithium-ion batteries can be recharged hundreds or even thousands of times before their capacity significantly diminishes. This makes them a popular choice for applications that require repeated charging and discharging cycles.
Another example is the lead-acid battery, commonly used in automobiles. Lead-acid batteries can be recharged by the vehicle's alternator while the engine is running, allowing the battery to store electrical energy and provide power to start the engine multiple times. These batteries are also used in backup power systems, such as uninterruptible power supplies (UPS), where they can be charged and discharged repeatedly to provide reliable standby power.
Accumulators play a crucial role in reducing waste generated by disposable batteries and promoting sustainable energy usage. With advancements in technology, the performance, capacity, and lifespan of accumulators continue to be improved, making them an integral part of modern energy storage solutions.
Reserve battery is a type of battery that is designed for long-term storage without the active electrode substance coming into contact with the electrolyte, or with the electrolyte being in a solid state. This unique design allows the battery to remain inactive and preserve its energy for extended periods, ranging from several years to decades. When the battery is required for use, it is activated by introducing a power source or a liquid electrolyte.
The purpose of reserve batteries is to provide reliable power in situations where immediate activation is not required, but long-term energy storage and stability are crucial. These batteries find applications in various industries, including military, aerospace, emergency backup systems, remote sensing devices, and other areas where a dependable power source is needed even after prolonged storage periods.
One practical example of a reserve battery is the lithium thionyl chloride (Li-SOCl2) battery. It consists of a lithium anode and a cathode made of thionyl chloride. The electrolyte in this battery is in a solid state, preventing chemical reactions and energy loss during storage. When the battery needs to be activated, a power source or a primary battery is connected to the reserve battery, causing the electrolyte to melt and enter the active state. This activation process enables the battery to deliver power efficiently and reliably.
Reserve batteries offer significant advantages in terms of long shelf life, low self-discharge rates, and high energy density. They are well-suited for applications where infrequent or sporadic power needs arise, such as in emergency lighting systems, electronic toll collection devices, or long-term storage of critical equipment. The ability of reserve batteries to retain their energy for extended periods, while still being capable of activation when required, makes them a valuable solution for ensuring reliable power supply in various professional settings.
Separator, in the context of batteries, refers to a crucial component.Its primary function is to prevent direct contact between the electrodes, thereby avoiding short circuits while still allowing the movement of ions through the electrolyte.
The separator is typically a porous material that possesses certain characteristics such as high porosity, mechanical strength, chemical stability, and low electrical resistance.
One common example of a separator material is polyethylene (PE) or polypropylene (PP) membranes. These materials are widely used in various battery technologies, including lithium-ion batteries, lead-acid batteries, and alkaline batteries. Simultaneously, it allows the transport of lithium ions, electrolyte ions, or other ionic species necessary for the electrochemical reactions to occur.
In lithium-ion batteries, the separator plays a critical role in preventing short circuits, thermal runaway, and other safety hazards. It acts as a physical barrier that inhibits the movement of solid particles or dendrites from the lithium metal or other active materials, which could cause internal short circuits and potentially lead to battery failure or even thermal runaway.
Additionally, separators can be surface-modified or coated with certain materials to enhance their performance and safety characteristics. For example, ceramic coatings or additives can improve the thermal stability and mechanical strength of the separator, thereby providing additional protection against thermal runaway under extreme operating conditions.
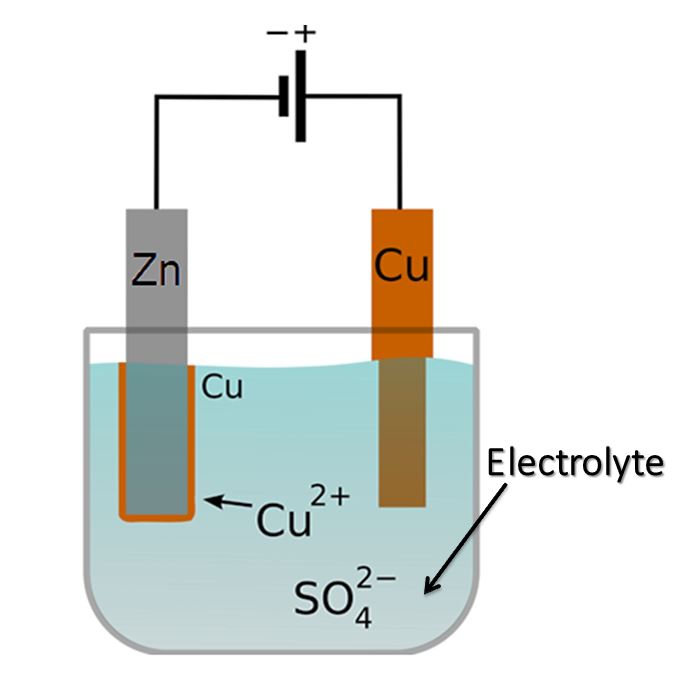
Electrolyte, in the context of chemical batteries and electrolytic capacitors, refers to a medium that facilitates the movement of ions and enables the normal operation of these devices. It serves as a conductive pathway for ions, allowing them to migrate between the positive and negative electrodes, thus completing the electrochemical reactions necessary for the device's functionality. The electrolyte also plays a crucial role in maintaining the reversibility of these chemical reactions.
In batteries, the electrolyte is typically a solution or a mixture that contains mobile ions. These ions can be positively charged (cations) or negatively charged (anions), depending on the type of battery. The electrolyte allows the flow of these ions between the electrodes, which are the sites where the electrochemical reactions occur.
One practical example of an electrolyte is the electrolyte used in lithium-ion batteries. It commonly consists of a lithium salt (such as lithium hexafluorophosphate or lithium perchlorate) dissolved in an organic solvent (such as ethylene carbonate or dimethyl carbonate).During battery operation, lithium ions (Li+) are released from the electrode materials. These lithium ions migrate through the electrolyte and interact with the electrode materials, facilitating reversible reactions that store and release electrical energy.
In electrolytic capacitors, the electrolyte is usually a conductive liquid or gel that fills the space between the electrodes. It allows the flow of ions, enabling the charging and discharging of the capacitor. The electrolyte in capacitors is specifically designed to have high ionic conductivity and low resistance to ensure efficient charge transfer.
It is important to note that the choice of electrolyte can significantly impact the performance, safety, and lifespan of electrochemical devices. Factors such as ion mobility, solubility, stability, and compatibility with electrode materials need to be considered when selecting an appropriate electrolyte for a specific application.
In summary, the electrolyte serves as a medium that provides ions necessary for the normal operation of chemical batteries and electrolytic capacitors. It enables reversible chemical reactions between the electrodes, allowing the storage and release of electrical energy. The selection and optimization of the electrolyte composition and properties are critical for achieving desired performance and reliability in electrochemical systems.
Electrolytic cell is an electrochemical device that establishes a circuit with an external power supply to drive a current through the electrochemical system, thereby inducing an electrochemical reaction to occur. It operates based on the principles of electrolysis, where the application of an electric current causes a non-spontaneous chemical reaction to take place.
The electrolytic cell consists of two electrodes—an anode and a cathode—immersed in an electrolyte solution. The anode is positively charged, while the cathode is negatively charged. When the external power supply is connected to the cell, it provides a potential difference that drives the movement of ions in the electrolyte.
During the electrolysis process, the positive ions (cations) are attracted to the cathode, where they gain electrons and undergo reduction reactions. Conversely, the negative ions (anions) migrate towards the anode, where they lose electrons and undergo oxidation reactions. These electrochemical reactions occur at the electrode-electrolyte interfaces.
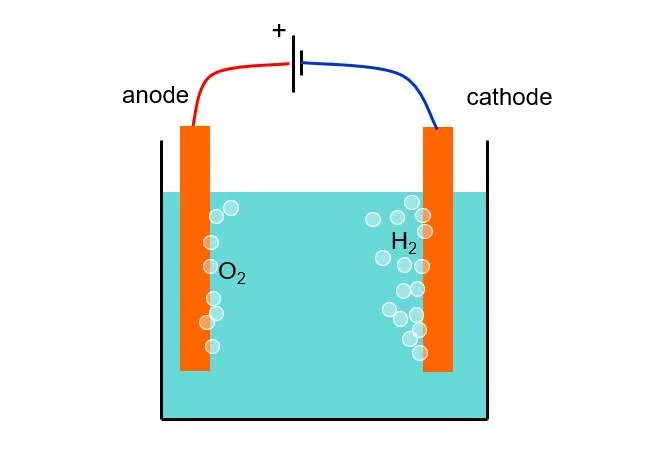
One practical example of an electrolytic cell is the electrolysis of water. When a direct current is passed through a water electrolytic cell, water molecules (H2O) are dissociated into hydrogen gas (H2) at the cathode and oxygen gas (O2) at the anode. The reduction half-reaction occurs at the cathode: 2H+ + 2e- → H2(g), while the oxidation half-reaction takes place at the anode: 2H2O → O2(g) + 4H+ + 4e-. This process enables the separation of water into its constituent elements.
The electrolysis of various compounds, such as salts, acids, or molten metals, can also be carried out in electrolytic cells. These cells have diverse applications, including electroplating, metal refining, production of chlorine and sodium hydroxide through the electrolysis of brine, and electrolytic production of aluminum from aluminum oxide.
Key characteristics of electrolytic cells include their ability to drive non-spontaneous reactions, their dependence on an external power source, and the separation of the electrodes by an ion-conductive electrolyte. They allow for precise control over the electrochemical processes and offer a wide range of industrial and scientific applications.
In summary, an electrolytic cell is an electrochemical device that utilizes an external power supply to force a current through an electrolyte and induce non-spontaneous chemical reactions. By understanding its principles and characteristics, electrolytic cells can be applied in various fields for processes such as electrolysis, electroplating, and industrial chemical production.
Ion-exchange membrane is a polymer membrane that contains ionic groups, enabling it to selectively transport specific ions in a solution. These membranes play a crucial role in various electrochemical processes, including electrolysis, electrodialysis, fuel cells, and ion exchange systems.
The principle behind ion-exchange membranes lies in their ability to facilitate the selective passage of ions based on their charge and size. The membrane is typically composed of a polymer matrix, such as fluorinated polymers or sulfonated polymers, with functional groups that can attract and interact with ions.
The ion-exchange process occurs when a solution containing different ions comes into contact with the membrane. The ionic groups within the membrane attract ions of the opposite charge, causing them to migrate through the membrane while blocking ions of the same charge. This selective ion transport allows for the separation, purification, or concentration of specific ions in a solution.
One practical example of an ion-exchange membrane is the Nafion membrane, widely used in fuel cells. Nafion is a perfluorosulfonic acid polymer that exhibits high proton conductivity. In a fuel cell, the ion-exchange membrane allows the transport of protons (H+) from the anode to the cathode, while blocking the passage of electrons. This selective ion transport enables the generation of electricity through electrochemical reactions.
Ion-exchange membranes also find applications in water desalination processes, such as electrodialysis. In this process, an ion-exchange membrane separates a feed solution into two compartments, allowing the selective passage of either positive or negative ions, depending on the membrane's characteristics. By applying an electric field, ions can be removed from the solution, enabling the desalination or purification of water.
Key characteristics of ion-exchange membranes include their ion selectivity, high ionic conductivity, mechanical stability, chemical resistance, and durability. These membranes can be tailored to specific applications by adjusting the type and concentration of ionic groups within the polymer matrix.
An ion-exchange membrane is a polymer membrane containing ionic groups that selectively transports ions based on their charge and size. By leveraging this selective ion transport, ion-exchange membranes are utilized in various electrochemical processes for ion separation, purification, and concentration. Their ion selectivity, conductivity, and durability make them essential components in technologies such as fuel cells, water desalination, and ion exchange systems.
Solid electrolyte interphase (SEI) refers to a thin passivation layer that forms at the solid-liquid interface between the electrode material and electrolyte during the initial charge and discharge cycles of a battery. It is a critical component in lithium-ion batteries and plays a crucial role in their performance, stability, and safety.
The formation of the SEI layer occurs due to reactions between the electrode material and the electrolyte components. These reactions can involve the reduction of electrolyte solvents and additives, as well as the decomposition of electrolyte salts. The SEI layer is primarily composed of organic and inorganic compounds resulting from these reactions.
The SEI layer acts as a protective film that covers the electrode material and helps prevent further reactions between the electrode and the electrolyte. It serves as a barrier that reduces the direct contact between the electrode and the electrolyte, thereby minimizing side reactions and improving the stability of the battery.
The SEI layer exhibits unique properties that contribute to its functionality. It is electronically insulating, preventing the passage of electrons and reducing unwanted side reactions at the electrode-electrolyte interface. However, it allows the transport of lithium ions, enabling the desired electrochemical reactions for battery operation.
The composition and characteristics of the SEI layer depend on various factors, including the type of electrode material, electrolyte formulation, cycling conditions, and battery chemistry. The formation and evolution of the SEI layer have a significant impact on the battery's performance, capacity, cycling stability, and safety.
One practical example of the SEI layer is observed in lithium-ion batteries using graphite as the anode material. During the initial cycles, the electrolyte reacts with the graphite surface, forming a stable SEI layer mainly composed of lithium carbonate (Li2CO3), lithium oxide (Li2O), and other organic compounds.
It is important to note that the SEI layer is not permanent and can evolve over time due to continuous cycling and aging of the battery. It may undergo chemical and structural changes, leading to its degradation or alteration in thickness and composition. The understanding and control of the SEI layer's formation and behavior are crucial for the development of advanced battery technologies with improved performance, lifespan, and safety.
In summary, the SEI is a passivation layer that forms at the solid-liquid interface of a battery, primarily in lithium-ion batteries. It acts as a protective film, electronically insulating the electrode material while enabling the transport of lithium ions. The composition and properties of the SEI layer impact battery performance and stability, making it a critical aspect of battery research and development.
NEWARE TECHNOLOGY LLC
755 Ames Avenue, Milpitas, CA, USA, 95035












