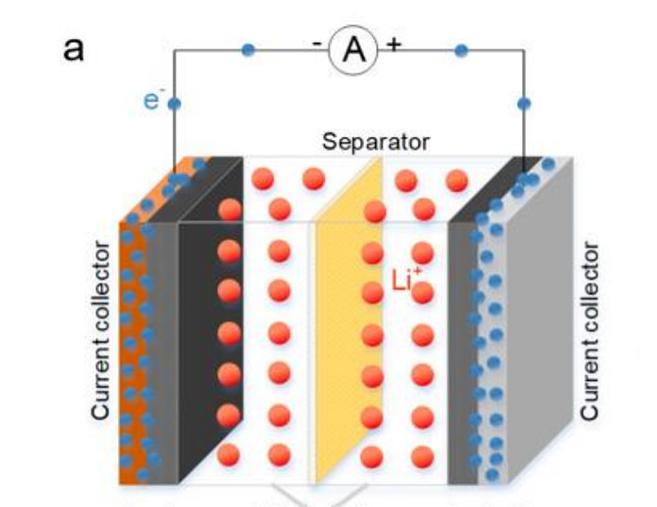
A current collector is an essential component in lithium-ion batteries that not only carries the active material but also collects and outputs the current generated by the electrode's active material. It helps reduce the internal resistance of lithium-ion batteries and improves their Coulombic efficiency, cycling stability, and rate performance.
Ideally, the ideal current collector for a lithium-ion battery should meet several criteria:
(1) high electrical conductivity, (2) good chemical and electrochemical stability, (3) high mechanical strength, (4) compatibility and strong bonding with the electrode's active material, (5) affordability and availability, (6) lightweight.
However, in practical applications, different current collector materials still have their own issues, making it challenging to fulfill all the aforementioned multiscale requirements. For example, copper is prone to oxidation at higher potentials, making it suitable as a current collector for negative electrodes. On the other hand, aluminum, when used as a current collector for negative electrodes, faces corrosion issues, making it more suitable as a current collector for positive electrodes. Currently, materials such as copper, aluminum, nickel, stainless steel, metal composites, carbon, and other semiconductor materials are used as current collectors in lithium-ion batteries.

Copper is an excellent metal conductor with high electrical conductivity, second only to silver. It has the advantages of abundant resources, affordability, and good ductility. However, considering that copper is prone to oxidation at higher potentials, it is commonly used as a current collector for negative electrode active materials such as graphite, silicon, tin, and cobalt-tin alloys. Common copper current collectors include copper foil, foam copper, copper mesh, and three-dimensional nanostructured copper arrays.

Copper foil can be further classified into rolled copper foil and electrolytic copper foil based on the production process. Compared to electrolytic copper foil, rolled copper foil has higher electrical conductivity and better elongation, making it suitable for lithium-ion batteries that do not require high bending. Research shows that increasing the surface roughness of copper foil helps improve the bonding strength between the current collector and the active material, reduce the contact resistance between the active material and the current collector, and consequently improve the battery's rate discharge performance and cycling stability.

Foam copper is a three-dimensional porous material similar to a sponge, characterized by its lightweight, high strength, and large surface area. While silicon and tin are promising negative electrode active materials with high theoretical capacity, they suffer from significant volume changes and pulverization during cycling, which severely impact battery performance. Research indicates that foam copper current collectors can suppress the volume changes and mitigate the pulverization of silicon and tin negative electrode active materials, thereby improving battery performance.

Although the electrical conductivity of aluminum is lower than that of copper, aluminum wire only requires half the mass of copper wire to transport the same amount of charge. Therefore, using aluminum current collectors helps increase the energy density of lithium-ion batteries. Additionally, aluminum is more cost-effective compared to copper. During the charge/discharge process of lithium-ion batteries, an aluminum foil current collector develops a dense oxide film on its surface, enhancing its corrosion resistance. As a result, aluminum foil is commonly used as a current collector for the positive electrode in lithium-ion batteries. Similar to copper foil current collectors, surface treatments can improve the surface characteristics of aluminum foil. Direct current etching creates a honeycomb-like structure on the aluminum foil surface, leading to tighter bonding with the positive electrode's active material and improving the electrochemical performance of lithium-ion batteries. However, in practice, aluminum current collectors often suffer from severe corrosion due to the breakdown of the surface passivation film, resulting in deteriorated performance of lithium-ion batteries. Therefore, optimizing the surface treatment of etched aluminum foil is necessary to enhance its corrosion resistance and form a more stable passivation film.

Nickel is a relatively inexpensive metal with good conductivity and stability in acidic and alkaline solutions. Therefore, nickel can be used as both a positive and negative current collector. Positive electrode active materials such as lithium iron phosphate, nickel oxide, sulfur, and carbon-silicon composites can be paired with nickel current collectors.

Nickel current collectors are typically available in two forms: nickel foam and nickel foil. Nickel foam has well-developed pores, which increase the contact area between the active material and the current collector, reducing the contact resistance. However, when using nickel foil as an electrode current collector, the active material may detach with an increasing number of charge/discharge cycles, affecting battery performance. Surface pretreatment techniques, such as etching the surface of the nickel foil, can enhance the bonding strength between the active material and the current collector.
Stainless steel is an alloy steel containing elements such as nickel, molybdenum, titanium, niobium, copper, and iron. It has good conductivity and stability, making it resistant to corrosion by weak corrosive media such as air, steam, and water, as well as strong corrosive media such as acids, alkalis, and salts. Stainless steel can form a passive film on its surface, protecting it from corrosion. Additionally, stainless steel can be processed to be thinner than copper, making it cost-effective, easy to manufacture, and suitable for mass production. Stainless steel can be used as a positive or negative electrode current collector, with common types being stainless steel mesh and porous stainless steel.
Stainless steel mesh has a dense structure. When used as a current collector, its surface is enveloped by the electrode active material, minimizing direct contact with the electrolyte. This reduces the occurrence of side reactions and improves the battery's cycling performance.
To fully utilize the active material and improve electrode discharge capacity, a simple and effective method is to use a porous current collector.
Using carbon materials as positive or negative electrode current collectors can avoid the corrosion of the electrolyte on metal current collectors. Carbon materials have advantages such as abundant resources, easy processing, low resistance, environmental friendliness, and low cost. Carbon fiber cloth, with its excellent flexibility, conductivity, and electrochemical stability, can be used as a current collector in flexible lithium-ion batteries. Carbon nanotubes are another form of carbon current collectors that offer the advantages of lightweight and significantly increased energy density compared to metal current collectors.
In addition to single current collectors such as copper, aluminum, nickel, stainless steel, and carbon, composite current collectors have gained attention in recent years. Examples include conductive resins, carbon-coated aluminum foil, and titanium-nickel shape memory alloys.
Conductive resin current collectors are composite materials made by combining conductive fillers (such as graphite and carbon black) with polymer resin matrices like polyethylene (PE) and phenolic resin (PF). These composite current collectors have been studied for their physical and chemical properties. Graphene, a unique two-dimensional carbon material with high electrical conductivity, surface area, and mechanical strength, can replace graphite as the negative electrode active material or be used as a current collector material.
Titanium-nickel shape memory alloys are binary alloys composed of nickel and titanium. They can undergo a reversible phase transformation between two different crystal phases with changes in temperature or applied pressure. By changing their phase, titanium-nickel shape memory alloys can suppress the volume change of the active material during charge/discharge processes, improving the battery's cycle life.
NEWARE TECHNOLOGY LLC
755 Ames Avenue, Milpitas, CA, USA, 95035












